What is the Best Zeolite?Easy Find Good Quality
Natural vs. Synthetic type,It was made by an aluminosilicate framework’s function of adsorbent and cation exchange capacity. It was made powdered,granules.
What is the Best Zeolite? Natural Zeolite vs Synthetic Zeolite
Table of Contents
Structure Of Bulk Zeolite Clinoptilolite
Natural vs Synthetic Zeolites, which is one of the lists of zeolites. It has major different tetrahedron structures, properties, and facts. It was manufactured by a porous crystalline aluminosilicate framework between silica (SiO4) and alumina (AlO4). The zeolite by synthesized processing was made and used for over 30 years in industrial applications. More and more professional engineers made synthetic types in their specialty craft. They possess many best ion-exchanged, catalytic, and molecular sieving properties, which make them economical mineral products.
Currently, there are over 58 different crystals structures of natural types, each having a unique particle size, adsorption, filtered capabilities, ion-exchanged, and specific applications. As a matter of fact, it includes stilbites, clinoptilolite, mordenite, chabazites, phillipsite, analcime, and laumontites. Its form with many different kinds of structures, which have large highly porous in a regular arrangement, and are roughly small molecules within the same particle size. In this image, Zeolite is trapped in the cages between sodium, potassium, magnesium, and hydrated molecules.
Function of Zeolite Clinoptilolite
High-purity natural clinoptilolite has the best ion-exchanged capacity. It can be used as an excellent soil amendment for soil and crops, as well as an odor adsorbent for ammonium, toxins, and heavy metals in animal bodies. NH4+ is a form of nitrogen available to both fruit and trees, based on clinoptilolite has a unique high affinity for NH4+. Once clinoptilolite absorbs more quantity of Ca2+ from natural phosphate stone, thereby slow-released ammonium ions and phosphate by step.
clinoptilolite we said is insoluble in the river. it can better hold additional ammonium and more nutrients in the crystal formers, and release nutrients slowly for a long time according to plant needs. It main works in agriculture and aquaculture. Suggest reading zeolite TDS / MSDS. Interesting, Natural zeolite is an economic pure nature mineral used in swimming pool applications. Customers change sand to natural zeolites in a pool filter. It is a best replacement way. Some families use it as cat kitty sand (kitty litter) at home, and it adsorbs animal waste odor.
The benefits of zeolites Are as below
The synthetic zeolite’s powerful adsorbing and effective cation exchange capacity stability is better than clinoptilolite except ignoring material cost. They saved nutrients input or pollutants reduction. The highly porous forms and channels that enter them contain moisture molecules. however, It also forms three-dimensional pore spheres to do cations exchange.
- 100% Organic clinoptilolite adsorbed by effective cation ion-exchange capacity (CEC)
- Best Fertilizer and animal feed nutrients were absorbed into the porous structure of organic clinoptilolite sand
- Clinoptilolite is used as a safe and natural desiccant for dog and cat litter. It is used as water filter media to solve the unacceptable smell of animal ordure. It also is ammonia remover to solve the same situation in the aquarium as fish and shrimp.
Natural Vs. Synthetic Zeolite’s Key Advantage
What Is The Major Difference?
- Generally, The ratio of silica to alumina in zeolite clinoptilolite is 5:1, and the ratio of synthetic zeolite is 1:1.
- Natural types are processed from the natural rocks of the Chinese volcano stone but synthetic types are made from energy-consuming chemicals.
- Zeolite powder will not decompose in a weakly acidic environment, while synthetic type will do. The natural structure contains more acid-resistant silica, which binds its structure together. zeolite powder is widely used for soil amendment and feed additives in agriculture.
How Do Natural Vs. Synthetic Zeolite Work
Our R&D dept focue the working principle of zeolite absorption, that the unique alumino-silicate framework of clinoptilolite adsorbs moisture and nutrients till the plant uses them. This result is similar to a common sponge.
| 100% high pure content | cation exchange capacity (CEC) |
| all natural clinoptilolite | about 80 to 120 meq/100g |
| all pure synthetic zeolite | about 180 to 250 meq/100g |
As shown above, most economical absorbents from nature focus on CEC and pure. Clinoptilolite has excellent and efficient adsorption capacity, so it has become a valuable mineral fertilizer. Not only It can effectively slow-release more nutrients to plants, but also it prevents them to waste soil nutrient loss. Farmer use can make plants and crops more nutritious in long term.
Synthesized zeolite materials include liquid alkali, silicate, aluminum, etc. These manufacturers can also produce structures that do not appear in nature. Synthetic type we said is a micronized type. Engineers use stabilized synthetic raw material to make it in a uniform and high-purity state.
Formula and Uses of Synthetic Zeolite
Firstly, the sodium zeolite formulation is Na 2Al 2Si 3O 10·2H2O. In the early days, it was formed naturally. but technical scientists have been able to synthesize sodium zeolites. These cation ion-exchanged zeolites have different acidity and catalyze a variety of acid catalysts. It is transformed into other minerals under long-term weathering, hot temperature reactions, or abnormal conditions. Na-A type is a well-known Synthetic Zeolite. Anten chemical is a professional supplier of Synthetic Zeolite in the world.
As we have said, Natural minerial supplied are unlimited, because silica material and alumina material are the most abundant mineral components in the world. Engineers use it as an ion-exchange builder in commercial and domestic softening water, water purification, and other catalytic applications.
Secondly, Na-A type is use as softening water builder in the household industry. They use catalysts and adsorbents to separate gas odor molecules by molecular sieve.
Although molecular sieves (synthetic zeolite) include the below type in the market, new types of molecular sieves are made in the future.
Application: Soil Amendment, Animal Feed, Aquaculture, Wastewater, Water Filter, Heavy Metal Removal, etc.
Application: Be used in desiccation of ethylene,propylene and ethanol
Application: Used in dehumid ification of oil-gas
Application: Used in air-purification filter and PSA for oxygen concentrator
Application: Be regenerated for re-use by pressure swing purging, usually at elevated temperatures.
Application: Used in purification of air with air separation equipment,and separation of gas or liquid
Application: ZSM-5 is a high Mole Ratio of SiO2/Al2O3 additive. Super XFocus® (a low-rare-earth SOx reduction additive) provide refiners with the most cost-effective solutions to reduce SOx emissions from their FCC units.
Application:
Application: Be used as a builder in Catalyst.
Thirdly, and most importantly, Zeolite powder from us is 100% pure natural and organic material from Chinese volcanic rock. It is a three-dimensional crystal structure built from the elements aluminum, oxygen, and silicon with alkali or alkaline-Earth metals.
Moreover, Our products include powdered, granules, and pellets with sand, but don’t make liquid zeolite for medical applications. Our resources’ clinoptilolite is a suitable material for animal feed, agricultural, and aquaculture use. In conclusion, Clinoptilolites are one of the most usual natural minerals.
A new application of zeolite is body detox, used as a cancer drug in medical care. A new lab submitted data from animals and the human body because researchers use this substance is injected it directly into the tumor. FDA doesn’t have zeolites as a cancer drug in human clinical trials, and the government has not approved zeolite supplements as safe or effective at present. So all patience must check zeolite warnings from health doctors.
Where Is The Mineral Source Of The Natural Clinoptilolite?
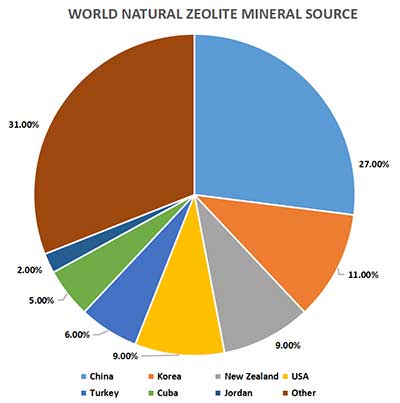
In Figure.1 we analyze the result. many countries developed bulk clinoptilolite capacity in the world. It includes China, Korea, New Zealand, the USA, Turkey, etc. clinoptilolite developed cost of Korea is highest. China is the largest manufacturer in the world. We saw fast market demand for more uses and new applications. This has caught the attention of many buyers.
We provide organic zeolite powder in China and awarded more testimonials of clinoptilolite powder, pellet, and granules over years. We have more professional experience in many applications.
- removing heavy metals
- binding agent of skin cream and detergent
- human body detox for medical
- adsorption of aquaculture pool filter
- water filter media
- filler of grass infill and golf turf
It is a magnet that can hold cations. Such as heavy metal, ammonia, low-level radioactive elements, toxins, several odors, petrochemicals, many different types of gases, and a multitude of various solutions. It is a highly porous sponge with a large surface area. That can absorb moisture up to 40% of its weight. The characteristic of the mineral lies in its cage structure. It’s aluminum and silicon content which enables the mineral a high cation exchange capacity.
In addition, They follow different applications to introduce different mesh size clinoptilolite. It includes powder, pellet, granules, sand, or small rocks. like clinoptilolite granules can’t match with swimming pool filters. Micronized zeolite isn’t suitable for high speed and high-pressure filters, because it has good dispersibility in swimming pool water. Small size clinoptilolite rocks don’t disperse in pond water. We use it for saltwater recycled in order to solve more wastewater. We ask what is zeolite. The above information is our answer.
Please contact us to reference the below info. I would support you.

